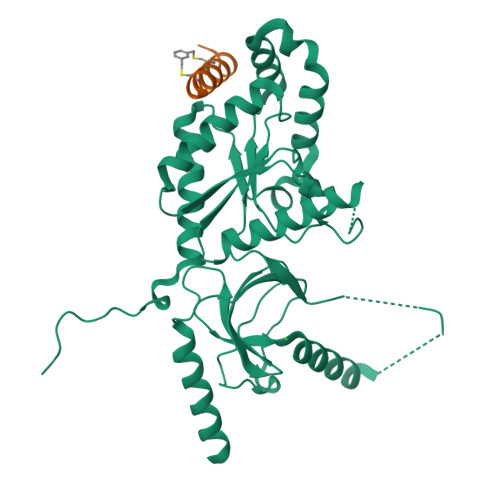
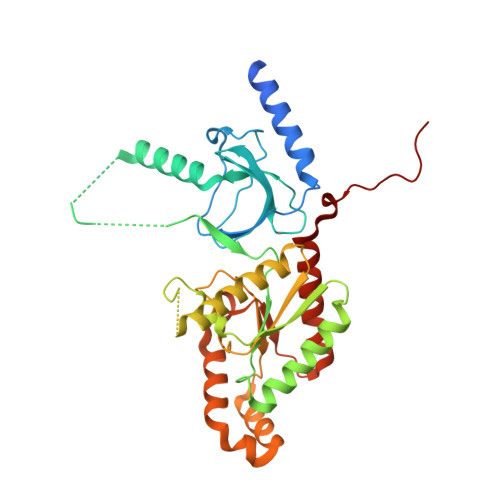
Stapled Voltage-Gated Calcium Channel (CaV) alpha-Interaction Domain (AID) Peptides Act As Selective Protein-Protein Interaction Inhibitors of CaV Function.
Findeisen, F., Campiglio, M., Jo, H., Abderemane-Ali, F., Rumpf, C.H., Pope, L., Rossen, N.D., Flucher, B.E., DeGrado, W.F., Minor, D.L.(2017) ACS Chem Neurosci 8: 1313-1326
- PubMed: 28278376
- DOI: https://doi.org/10.1021/acschemneuro.6b00454
- Primary Citation of Related Structures:
5V2P, 5V2Q - PubMed Abstract:
For many voltage-gated ion channels (VGICs), creation of a properly functioning ion channel requires the formation of specific protein-protein interactions between the transmembrane pore-forming subunits and cystoplasmic accessory subunits. Despite the importance of such protein-protein interactions in VGIC function and assembly, their potential as sites for VGIC modulator development has been largely overlooked. Here, we develop meta-xylyl (m-xylyl) stapled peptides that target a prototypic VGIC high affinity protein-protein interaction, the interaction between the voltage-gated calcium channel (Ca V ) pore-forming subunit α-interaction domain (AID) and cytoplasmic β-subunit (Ca V β). We show using circular dichroism spectroscopy, X-ray crystallography, and isothermal titration calorimetry that the m-xylyl staples enhance AID helix formation are structurally compatible with native-like AID:Ca V β interactions and reduce the entropic penalty associated with AID binding to Ca V β. Importantly, electrophysiological studies reveal that stapled AID peptides act as effective inhibitors of the Ca V α 1 :Ca V β interaction that modulate Ca V function in an Ca V β isoform-selective manner. Together, our studies provide a proof-of-concept demonstration of the use of protein-protein interaction inhibitors to control VGIC function and point to strategies for improved AID-based Ca V modulator design.
Organizational Affiliation:
Molecular Biophysics & Integrated Imaging Division, Lawrence Berkeley National Laboratory , Berkeley, California 94720, United States.